Background
Classic Hodgkin lymphoma (cHL) and aggressive non-Hodgkin lymphoma (NHL) account for an estimated 0.5% and 4.1% of all new cancer diagnosis in the US in 2023, respectively. At time of diagnosis, approximately 43% of cHL and 53% of NHL are staged as advanced disease (stage III-IV). While these diseases are considered curable, standard treatments involve intensive combination chemotherapy that may preclude certain patients from receiving optimal therapy. One option to minimize risks is to use prephase cyclophosphamide (pCy) as an initial treatment in aggressive lymphomas. There is however limited data on the utility, adverse effects, and outcomes of this approach. Here we present data on our institutional experience of employing pCy for patients ineligible for intensive chemotherapy.
Methods
We performed a single institution, retrospective chart review of patients with history of cHL and NHL that received cyclophosphamide (Cy) as an initial chemotherapy during hospitalization. Lymphoma subtype, medical history, baseline bloodwork at time of treatment initiation, outcomes including transplant, relapse data and overall survival were collected. Fifty patients received pCy at 200 mg/m2 with the remaining four receiving 100 mg/m2, 150 mg/m2, or 300 mg/m2 based on provider preference. Corticosteroids, including prednisone, dexamethasone or methylprednisolone, were administered simultaneously with varying duration and dosage. We analyzed hepatic and kidney injury at the time of diagnosis, after initiation of pCy and time to recovery. Acute kidney injury (AKI) was defined as an increase in creatine more than 30% from baseline and hepatic injury was defined based on Common Terminology Criteria for Adverse Events (CTCEA) version 5. Charlson scores for all patients were collected.
Results
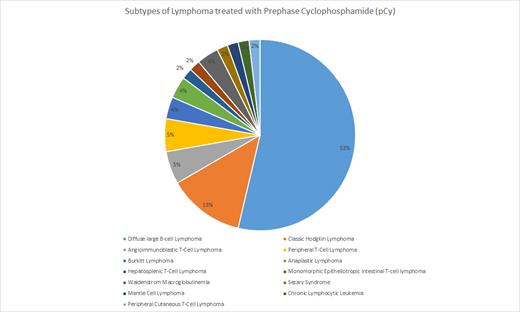
Fifty-four patients received pCy as a bridge to possible definitive therapy. The most common diagnosis was diffuse large B-cell lymphoma (DLBCL) and cHL, with 29 (53.7%) and seven (13%) patients, respectively (Figure 1). The median age was 56 years (18-84) with 21 (39%) patients >65 years. Advanced stage was observed in 49 (90.7%) patients. Four patients required treatment for tumor lysis syndrome (TLS) prior to initiation of pCy. Nineteen (35%) patients had AKI and 15 (28%) patients had hepatic injury at the time of initial presentation. Five patients experienced AKI from pCy with an average time to recovery of 11.2 days. Six patients, with only two having concurrent AKI, had hepatic injury from pCy with an average time to recovery of 8.5 days. PCy allowed 42 (78%) patients to undergo definitive treatment in respect to their lymphoma subtype. Eight (14.8%) patients went on to receive a consolidative autologous transplantation (HSCT) after reaching a remission from definitive therapy. At the time of our analysis, 30 (55%) patients are deceased which includes four patients that underwent HSCT. Of the 30 deceased, 10 patients were transitioned to comfort care during the admission after receiving pCy. Median overall survival is 370.5 days.
Conclusion
Based on our institutional experience, pCy is a reliable method to salvage patients with aggressive lymphomas that are not candidates for intensive chemotherapy. In our analysis, pCy allowed 78% of patients to receive definitive therapy for their respective lymphoma. In general, patients receiving pCy required hospitalization related to disease burden and pCy represents an effective and cost-efficient bridging strategy to outpatient treatment. Cyclophosphamide has a broad indication across hematologic malignancies and is a well-tolerated chemotherapy with a limited side effect profile. The modest dosing in combination with corticosteroids allows initial disease control and provides the ability to start definitive therapy in a timely manner. Additionally, we feel that pCy should be considered when designing clinical trials in an effort to promote enrollment of the “real-world” patient.
Disclosures
Kota:Incyte: Research Funding; Pfizer: Honoraria; Kite: Honoraria; Novartis: Honoraria. Cortes:Takeda: Consultancy, Honoraria; Gilead: Consultancy; Forma Therapuetic: Consultancy; Abbvie: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Keruakous:BMS: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal